THE CHALLENGE: OVERWHELMING COMPLEXITY
The client: large biotechnology company.
Clinical samples traverse a complex path. Whether the samples originate from sites halfway across the planet or across the street, clinical research teams are faced with the challenge of monitoring the status of biospecimens across multiple sites, labs, and repositories at multiple phases of development. Complex sample operations, disparate vendors, and inconsistent metadata across labs are common challenges sponsors face when keeping track of samples across clinical programs.
Complexity was the challenge one large biotechnology company faced as its clinical operations and translational science teams sought to track clinical samples across dozens of studies, dozens of specialty labs, and multiple central labs. The client previously used a custom-built, internally developed solution that enforced a rigid deployment approach and was unable to accommodate a variety of data types and data structures.
THE SOLUTION: QUARTZBIO® vSIM SOLUTION
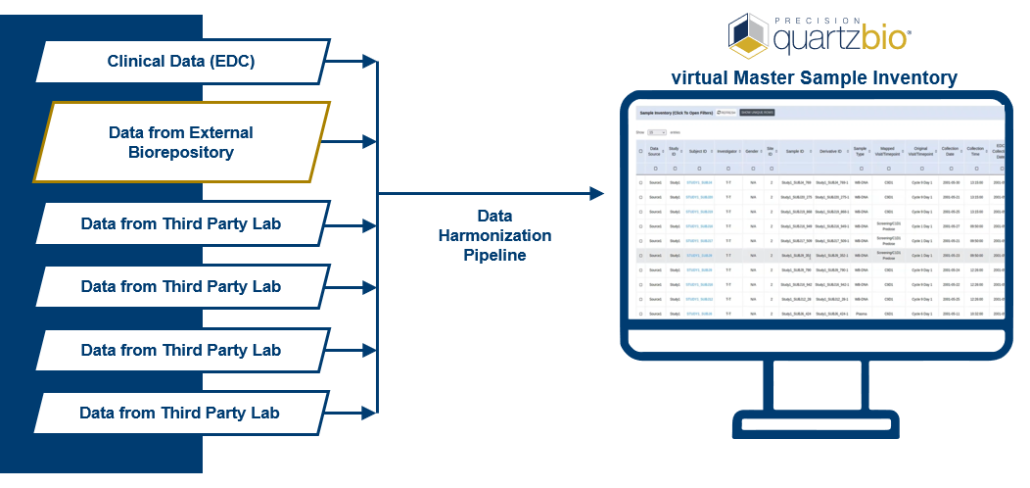
When the client began to search for a new platform to replace its homegrown solution, QuartzBio’s virtual Sample Inventory Management solution (vSIM) presented itself as a more capable alternative solution. The client partnered with QuartzBio experts to ramp up quickly and provide immediate results.
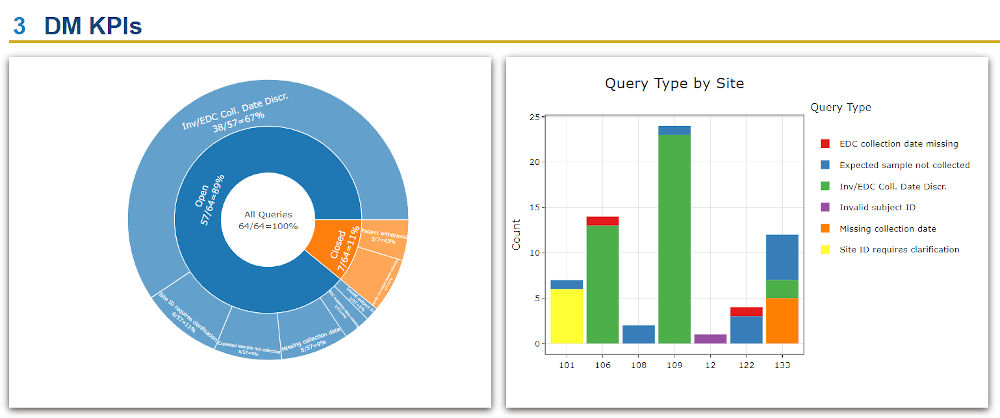
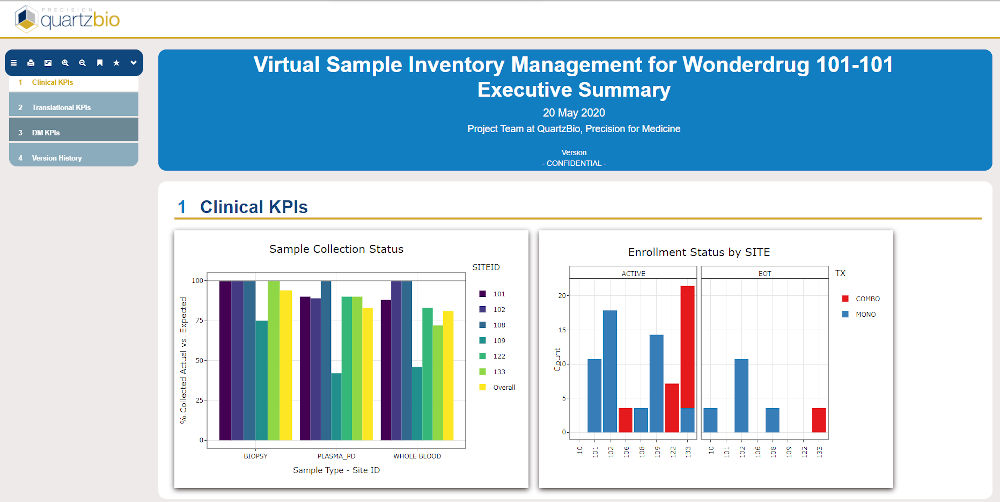
- The vSIM solution enabled the client’s teams to evaluate the status of samples on study and easily track consent, collection, and processing of samples. This made it easier to manage queries and identify discrepancies or patterns of non-compliance.
- The client was able to start studies with new levels of speed and efficiency. Because the vSIM solution was “source-agnostic” in its approach to data collection, the client now had the flexibility to accommodate a variety of data types, formats, and structures. Ultimately, this range of seamless data consumption and integration enabled the client’s teams to identify sample availability by patient, assay, and other parameters. The client no longer had to devote time and resources to anticipate and set up a multitude of data pipelines. Instead, teams were able to utilize a library of configuration-ready application programming interfaces (APIs) as well as connectors and data processing pipelines. This enabled teams to visualize and review key pre-analytical data, detect patterns of site-based noncompliance, and identify data discrepancies early.
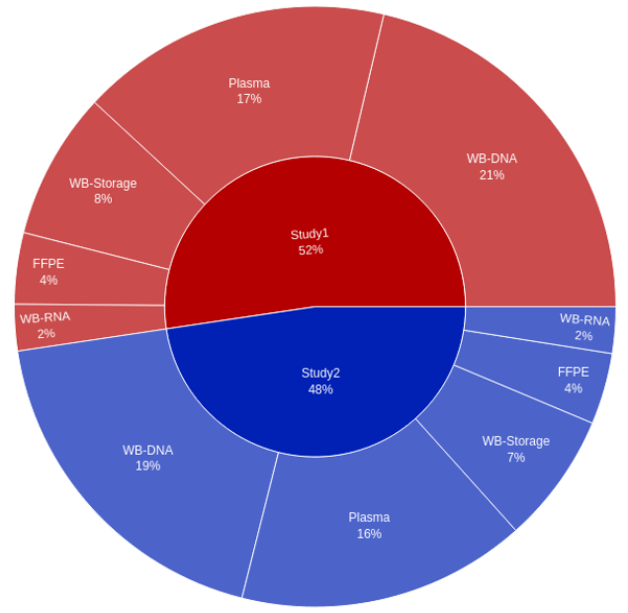
- The client was able to build versatile portfolio-level dashboards and provide unprecedented visibility into key performance indicators (KPIs) such as missing samples, samples with expiring/expired stability or consent dates, lab turnaround times, sample testing status, and other operational metrics to drive on-study decision making.
- Because the vSIM solution alerted the client to samples with imminently expiring consent and stability windows, the client was able to use over 500 samples before expiration to support current and planned studies.
Where sponsor teams previously lacked centralized visibility, they now possessed insight into the collection, processing, and storage status across multiple siloed source systems.
This powerful centralized visibility also had bottom-line ramifications on the client’s overall growth. Senior executives, including the CEO and CFO, were conducting serious discussions with another company that had identified it as a potential acquisition target and began auditing their inventory of valuable clinical biospecimens. Where requests for sample collection data had previously required extensive lead-time and resource allocation to perform, senior executive users now had real-time metrics at their fingertips to drive decision-making.
THE RESULTS: THE vSIM SOLUTION BECOMES THE “SINGLE SOURCE OF TRUTH”
QuartzBio’s vSIM solution quickly became a true game-changer for the client. By ingesting lab sample inventories, relevant electronic data capture (EDC extracts), sample manifests, electronic sample requisition forms (SRFs), and informed consent forms (ICFs), the client was ultimately able to create a virtual master sample inventory to track samples across every site, lab, and repository over their entire lifecycle. New growth opportunities emerged because vSIM powered a new level of flexibility and scalability to support everything from a single clinical program up to an entire portfolio of hundreds of studies across all phases of development – a single source of truth for all sample-related data.