Title: Critical Biomarker Operations KPIs Across Clinical Portfolios
Duration: 30 minutes
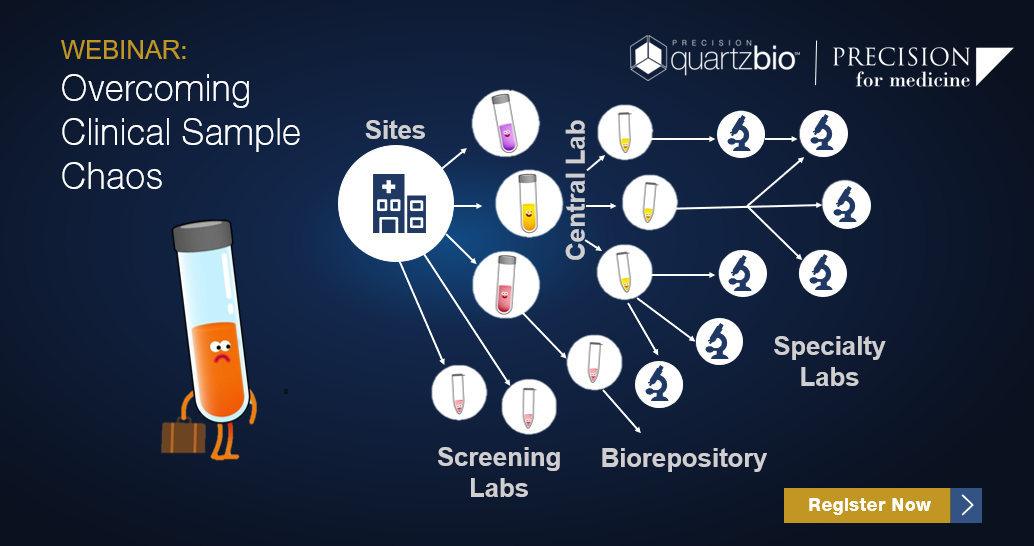
Clinical and biomarker operations teams face challenges with global site footprints, complex sample lifecycles, and inconsistent naming across sites and vendors.
Watch our webinar to learn how teams are monitoring key performance indicators (KPIs) for clinical sample status, quality, and data availability – on-study and across clinical portfolios.
Adam S. Brown, PhD of QuartzBio will share how teams are assessing key deliverables:
- Site performance including collection rates, collection quality metrics, and automated reporting for consent and protocol violations.
- Vendor operational performance such as adherence to contracted turnaround windows and time to analysis.
- Results data availability across assay modalities at study, cohort, patient, and sample levels – with comparisons of actual vs expected events, and forward-looking projections.
- Sample and derivative availability across active and completed studies, including in-depth profiling of viability and volume, consent status, and expiration dates.