Join QuartzBio at the SCOPE 2021 virtual conference for the “Clinical Biomarkers Operations and Innovation” track on March 2.
PRESENTATION: “Centralizing PK, Clinical, and Exploratory Biospecimen Data to Streamline Trial Operations & Intelligence”
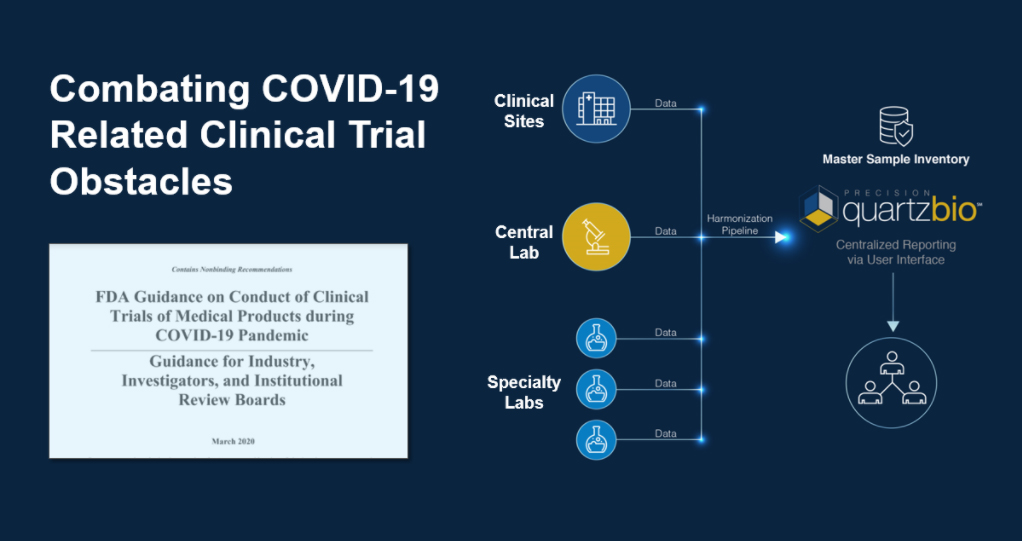
Clinical samples are frequently a logistical headache – managed too often in Excel files that are time-consuming to update, and quickly fall out of date. Modern biomarker-centric programs demand a focused strategy to provide timely visibility into sample availability and quality.
Dr. Tobi Guennel will share how clinical teams are leveraging technology to quickly and efficiently gain visibility into their clinical samples across sites, labs, and storage facilities.
Read more