In a recent article titled, “The Criticality of Specimen Management to Precision Medicine,” Brenda Yanak warned drug developers against minimizing the importance of biospecimens, so often overlooked during biomarker development.
Dr. Yanak is known in the Biospecimen and Biomarker Operations field for her transformative work in translating scientific strategy into clinical operations, via technology and innovation, across both biopharma and central labs. Her efforts have succeeded in drawing attention to biospecimens, which are central to diagnosis and treatment.
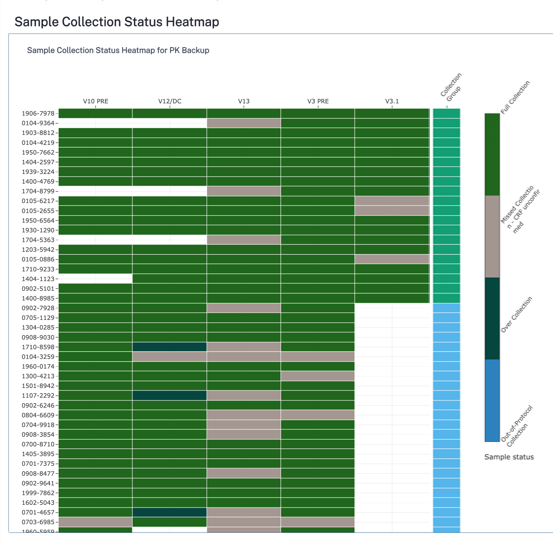
However, as she points out in this article, transparency in biospecimen chain of custody is frequently lacking, with responsibilities split among multiple organizations, leading to unclear processes, extended timelines, and inefficiencies.
Dr. Yanak’s article highlighted both challenges of current processes and specific ways to improve biospecimen management:
Challenges Present in Traditional Biospecimen Operations:
- Roles and responsibilities among various teams, both internal and external, are often unclear.
- Lack of transparency in biospecimen chain of custody contributes to extended clinical trial timelines.
- Manual tracking using Excel spreadsheets is common, leading to inefficiencies and potential errors.
Improving Biospecimen Management:
- There is a need for Quality Management System (QMS)-level specimen management processes.
- Technology solutions, such as QuartzBio’s virtual Sample Inventory Management (vSIM) solution, can streamline biospecimen management.
- Minimize manual tasks for fewer errors and increased speed.
- Standardization and collaboration are proposed to accelerate biomarker research and clinical trials in the precision medicine era.