The challenge: Maintaining clinical trial timelines dependent on vendor performance
A global biotechnology company with seven clinical-phase programs approached QuartzBio with a challenge shared by many organizations in the industry — overall assessment of clinical trial vendors and sites was time-consuming and unreliable, given global site footprints, complex sample lifecycles, and inconsistent naming across sites and vendors.
Tasked with preparing regular executive summaries, the company’s clinical and biomarker operations teams were spending significant time deriving trial-wide key performance indicators (KPIs), lacking visibility into vendor performance – and challenged to translate current experience into future study planning.
This lack of visibility was exacerbated by the absence of standard nomenclature and inconsistent data entry across vendors. Clinical operations teams were spending time manually mapping and reconciling data and metadata from multiple independent sources.
The QuartzBio approach: Continuously updated visibility through consolidated dashboards
The client implemented the virtual Sample Inventory Management solution, powered by the QuartzBio® Data Platform.
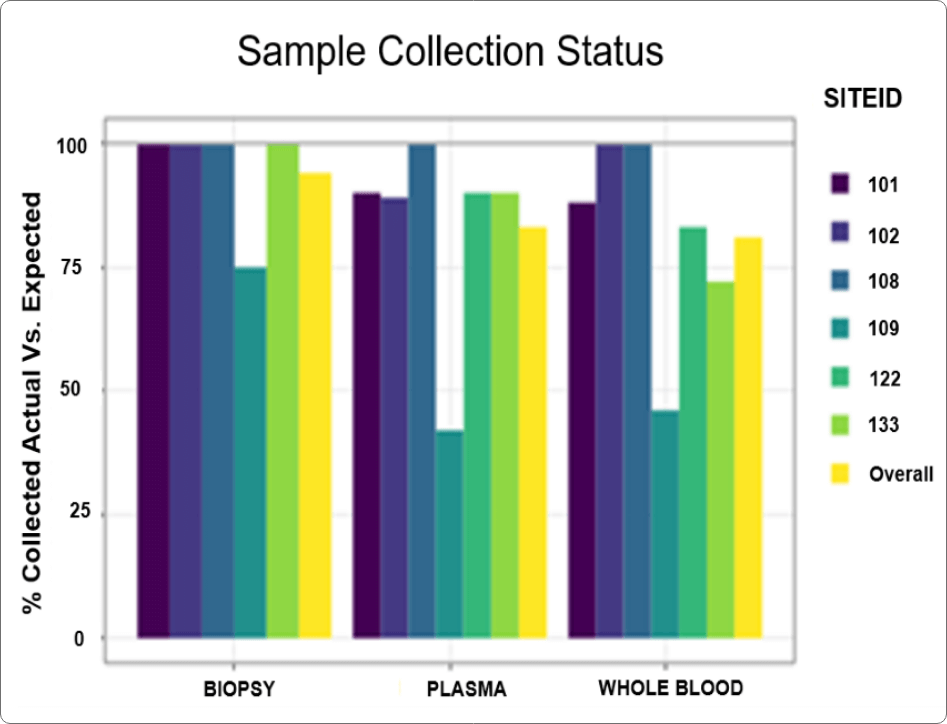
Using the platform’s built-in dynamic dashboards (examples shown in Figure 1), augmented by some bespoke reports custom-built by QuartzBio for the client, the clinical operations team was able to easily monitor and report on multiple vendor performance parameters:
- Turnaround times from sample collection to sample processing to test result generation
- Completion rate of samples tested within stability window
- Sample processing quality KPIs by site and vendor
- Rate of missed/incomplete baseline and on treatment collections
- Data discrepancies by site and vendor
- Protocol violations by site and vendor
- Consent violations by site and vendor
Core to QuartzBio’s approach is rigorous sample data integration. The virtual Sample Inventory Management solution automatically harmonizes sample information from multiple sites, vendors, and other sources, regardless of format or nomenclature. QuartzBio applies an adaptive data dictionary that learns and updates common nomenclatures over time, creating a flexible model. Using this model, it harmonizes the non-standard nomenclature across vendors and studies, enabling clear visibility and eliminating manual processes.
The outcome: Fast, accurate reports, timely decisions, course correction on-study
Empowered by 24/7 access to vendor performance, visibility into expected sample schedules, and full reconciliation oversight, the client’s clinical operations teams started using the QuartzBio Data Platform for generating visualizations to guide executives and inform investors.
These visualizations enabled the client to take action at specific sites and vendors that were underperforming. Armed with more reliable trends in sample schedules and turnaround times, the biomarker operations team could more accurately project data delivery timelines and anticipate when key decisions could be made.