The client: Clinical operations team at a mid-sized oncology biotechnology company.
The clinical operations teams at a mid-sized oncology biotechnology company approached QuartzBio as they sought ways to close sample information gaps that would appear during reconciliation.
Gaps in this process typically included the inability to report in a timely manner on:
- Missed collections
- Unexpected samples
- The status of samples and derivatives as they move onto third-party testing labs
The challenge: The sponsor team lacked global visibility into their expected sample schedule across all collection sites. Too much time was spent on manual reconciliation, with delays of three months costing the client significant time and resources.
In their existing reconciliation process, clinical operations leaders were often frustrated in their attempts to maintain robust global visibility. Generating reports was a time-consuming process that involved complex spreadsheets manually linking key information from the EDC, central labs, testing labs, and biorepositories to illuminate critical information like discrepancies or missed collections.
Without global visibility, the client had trouble generating reports continuously throughout the life of a study. New data was often unavailable, vendor-specific nomenclature was frequently inconsistent, and data structures were sometimes incompatible. Consolidated insight across sample collection and processing status, consent status, and sample and derivative quality information was difficult to obtain.
The solution: The sponsor team used the QuartzBio® virtual Sample Inventory Management (vSIM) Enterprise Software-as-a-Service (SaaS) solution to systematically monitor and identify:
- Gaps in sample data
- Issues with sample shipments
- Data discrepancies
- Consent violations
- Subject exclusions
The client’s clinical operations team worked with QuartzBio’s biospecimen operations experts and data engineers to deploy the vSIM solution and create a centralized, reliable source of sample information. This single solution closed the gaps that were hampering the reconciliation process.
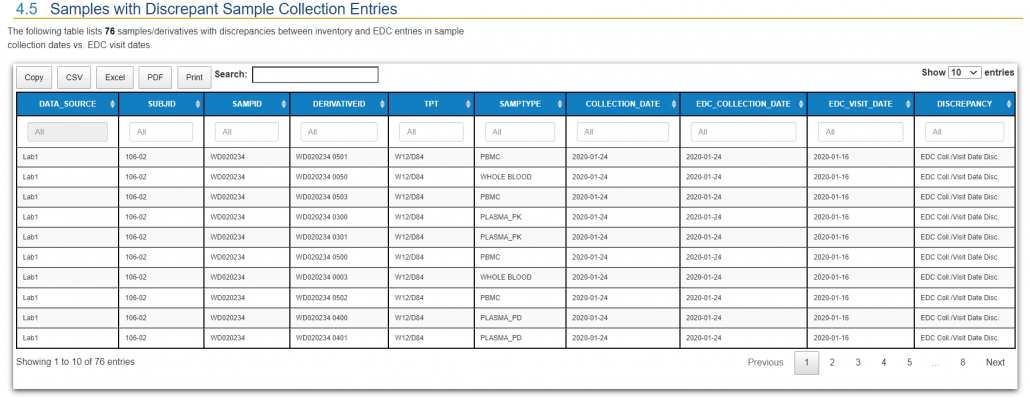
QuartzBio’s data ingestion and harmonization pipeline continuously integrated sample information from multiple sources. As this sample information was generated over the course of the study, the vSIM solution automatically performed edit checks and highlighted any data inconsistencies. For instance, teams could now see any direct reconciliation issues between the EDC and inventories at central labs, specialty labs, and biorepositories (Figure 1).

The outcome: Eliminating manual reconciliation processes with sponsor-centric visibility across the sample lifecycle
Reconciliation efforts typically have large gaps in sample information that is critical for sponsor teams to maintain robust global visibility across all collection sites. Clinical data management traditionally reconciles the EDC from other points of view (e.g., adverse events, concomitant medications, patient history, RECIST response evaluation, investigational product guideline (IP) compliance, and many others). The QuartzBio vSIM solution functioned as an additional tool pulling information together from the EDC, each lab, and each biorepository. The solution also incorporated the sample schedule from the protocol, including changes to the protocol as new patients were enrolled and the protocol was amended.
With reconciliation gaps reduced and closed, clinical operations teams now had sponsor-centric visibility into sample status across the lifecycle of collections, processing, testing, and storage. With a single day of delay in getting a new product to market costing $1-$8 million in sales (source), the benefits of more effective sample and biomarker operations providing a holistic view of samples on-study cannot be understated.