April 1, 2020 — The global pandemic caused by COVID-19 is presenting a public health crisis, and unprecedented challenges in the conduct of clinical trials. The quickly realized disruption has motivated FDA and EMA to issue guidance in direct response. As we continue to adapt to the new reality, we are seeing proactive monitoring of strained health care systems and potential for shipping disruptions as top of mind issues for Sponsors operating ongoing trials. This is especially true as Sponsors work to maintain R&D momentum and continue to serve patients – while adapting to an increasingly remote workforce requiring virtual collaboration.
COVID-19 is disrupting patient visits, sample collections and sample shipments globally – making it difficult to identify, track, and course correct deviations from a position of remote monitoring. Sponsors, CROs, and labs are especially prioritizing sample quality and logistics, making this a mission critical component now more than ever. Following the recent FDA and EMA guidance, fast-paced protocol and ICS amendments will be a necessity for ongoing clinical trials and the collection of samples from clinical sites and distribution of samples to central and specialty labs is of specific emphasis.
Outlined below, are 5 of the key considerations for maintaining and documenting clinical sample integrity in current and ongoing studies:
- FDA and EMA guidance requires sponsors to identify and document the impact of COVID-19 on ongoing trials. As one example, FDA expects specific information to explain the basis of missing data, including the relationship to COVID-19 for missing protocol-specified information (e.g. missed study visits or study discontinuations due to COVID-19).
- Sponsor trial management teams are transitioning to remote management, requiring innovative approaches to enable collaboration and support compliance.
- Sample shipments – particularly across borders – are increasingly unpredictable and may impact testing plans when samples are time sensitive.
- The combination of protocol amendments and potential transition of labs increases administrative burden for linking samples to correct ICF versions, to guide data generation strategies.
- Sample collection may increasingly be conducted on a remote basis, adding process change and operational complexity.
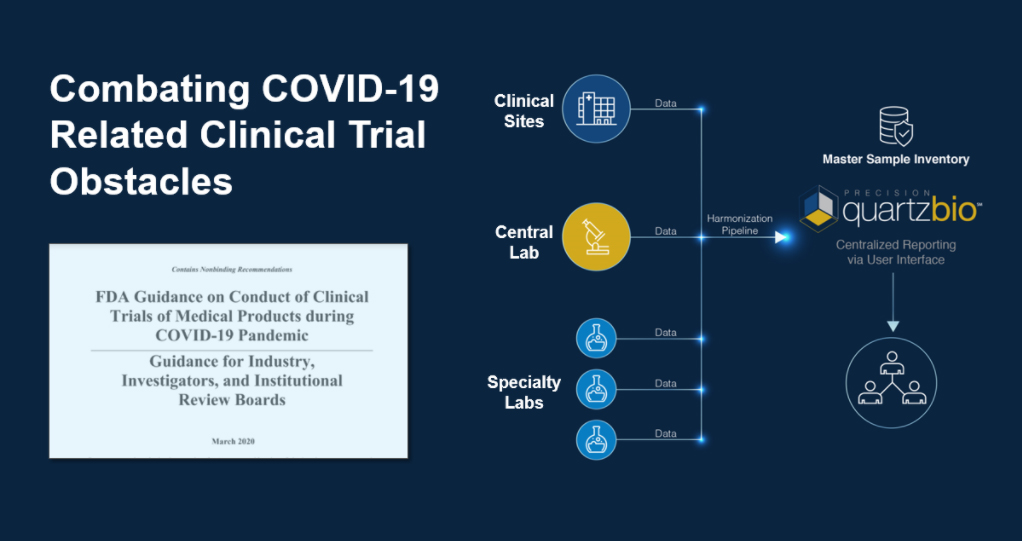
The QuartzBio team is rapidly deploying our cloud-based virtual Sample Inventory Management (vSIM) solution to support Sponsors responding to the current climate by providing:
- Consolidated, “master” sample inventory across a labs within a clinical trial to provide ongoing and comprehensive visibility into samples, including to identify site-specific quality issues which may increase risk of deviations.
- Collaborative, web-based platform to increase CRA and site monitoring efficiency via interactive and shareable dashboards.
- Rapid startup to support ongoing studies, leveraging data that is already available from vendors.
- Forward-looking visibility into expected sample collection to enable coordination with labs and continuous updates on actual vs. expected.
- Capture information regulatory agencies require for documentation of COVID-19 impacts.
The unpredictable and quickly evolving nature of the current situation poses a particular challenge to ongoing trials. Dynamic and creative solutions that support remote monitoring and compliance assurance (and which can be rapidly deployed) will be required to accompany clinical trials through this difficult period.

Author: Scott Marshall, PhD | Managing Director | QuartzBio, Part of Precision for Medicine

