Duration: 30 minutes
Register to watch on demand:
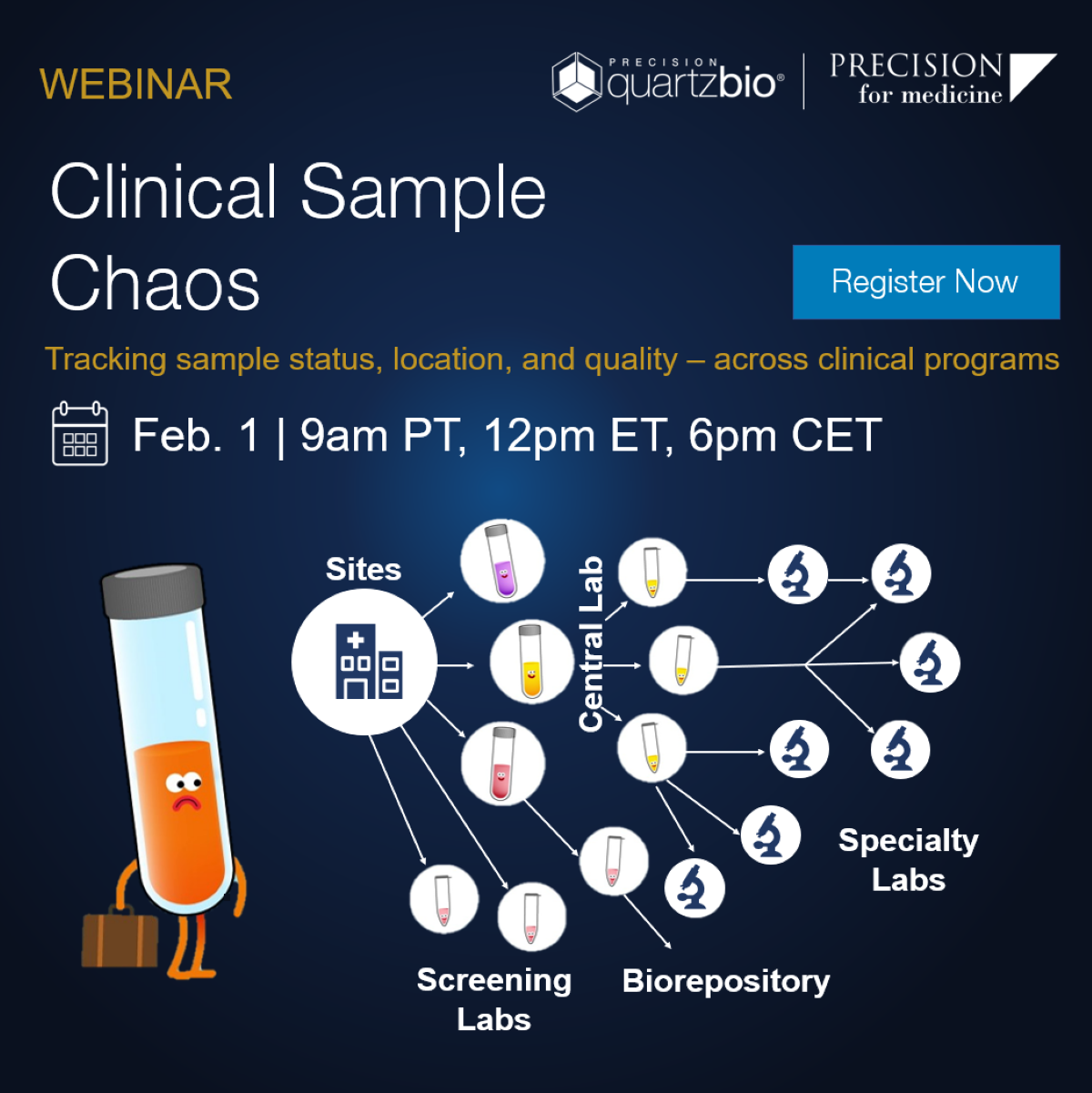
Complex sample operations, disparate vendors, inconsistent metadata across labs are common challenges sponsors face when keeping track of samples across clinical programs.
Join our webinar to learn how sponsor teams are creating consistent, portfolio-level approaches for tracking sample status and location and uncovering insights into vendor/site/country-level performance.
You’ll see how virtual Sample Inventory Management:
- Ensures the right samples get to the right labs on time for testing, with cross-program visibility into collection, derivatives, and processing.
- Tracks consent status, including optional consents, for all samples, so that testing is performed in compliance.
- Maintains insights into site and vendor performance across studies with rigorous, transparent quality control
- Ingests data from any source, as-is according to existing DTAs, and harmonizes nomenclature with a common data dictionary
- Enables discrepancy identification and query management across all vendors involved in sample lifecycles.
Who should join:
Clinical Operations teams, Biomarker Operations teams, Translational Research teams, Office of the CIO/CTO, Data Management teams
Watch Now >>
About the presenter:
QuartzBio, part of Precision for Medicine, delivers end-to-end SaaS solutions to accelerate drug development.

