Clinical sample management made easy with QuartzBio® vSIM and AI Virtual Assistant
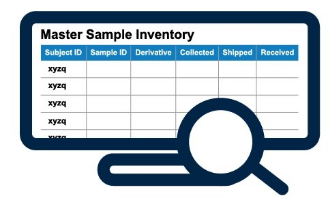
Master Sample Inventory (MSI)
- Identify missing samples (expected, but not collected)
- Identify out of protocol collections (collected, but not expected)
- Designed to handle different collection groups (cohorts, amendments, regions)
- Apply business logic to compare actual visit dates vs. expected visit dates
- View projected collections for a patient
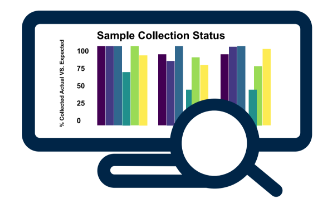
Sample Collection Monitoring & Compliance
- Identify missing samples (expected, but not collected)
- Identify out of protocol collections (collected, but not expected)
- Designed to handle different collection groups (cohorts, amendments, regions)
- Apply business logic to compare actual visit dates vs. expected visit dates
- View projected collections for a patient
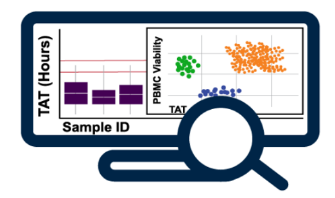
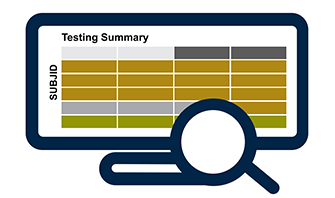
Testing Monitoring: Sample Testing & Stability Summaries
- Apply business logic to assess sample stability based on assay-specific criteria
- Flag sample expiration
- Visual & tabular outputs
Dynamic Dashboards & Enterprise Reporting
- User can create dynamic dashboards and widgets
- Enterprise Reporting:
- Report on sample collection summaries across studies
- Site- and vendor-level reporting across studies
Data Checks
- Automated standard data checks
- File-level structure and format checks
- Potential issues from subject IDs
- Potential issues from duplicate
- Sample IDs
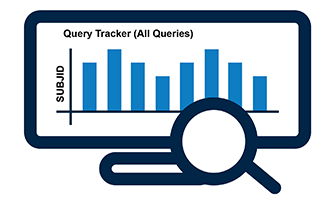
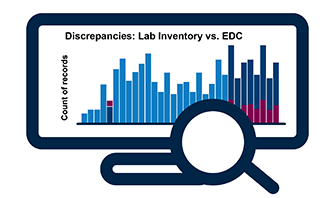
Data Reconciliation & Query Tracking
- Identify discrepancies between data that resides in any two source files
- Summary output in vSIM report
- Issues queries in the Query Tracker
- Automatically generated queries issued from various data checks and data reconciliation
- Automatically close resolved queries
- Users can resolve queries
- Users can download Query Tracker to manage queries with labs
Logistics: Sample Shipment Lists & Tracking
- Tabular view of available samples based on configurable business logic (e.g., sample status, sample stability, open queries, batch size)
- Identify samples eligible for shipping
- Shipment tracking module available in QuartzBio’s UI that links to courier databases (FedEx, Quickstat, and World Courier)
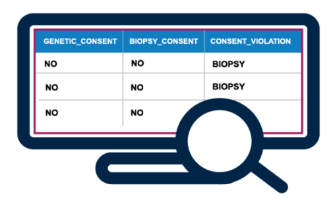
Consent Tracking
- Get visibility into consent status by linking digital indicators of consent to sample metadata
vSIM capabilities deliver sample intelligence. Add our AI Virtual Assistant for even faster insights.