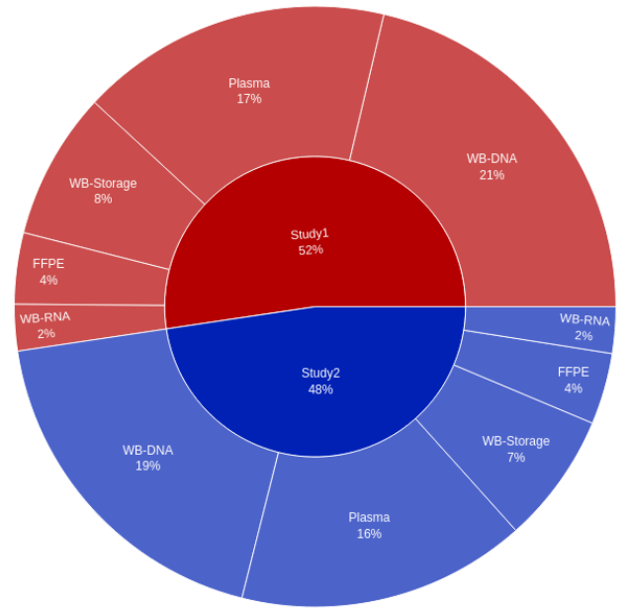
One of the core challenges faced by clients in biomarker-informed clinical trials is the integration of key sample and subject metadata derived from multiple independent sources. One case involved multiple immuno-oncology studies, with data coming from multiple sources, in multiple formats.
The QuartzBio® platform was deployed to create a Master Sample Inventory view, yielding answers to key on-study questions, such as:
- How does the number of available plasma samples compare to the number of available FFPE samples for Study X?
- What proportion of samples have data available? What’s the breakdown of data availability by sample type?

