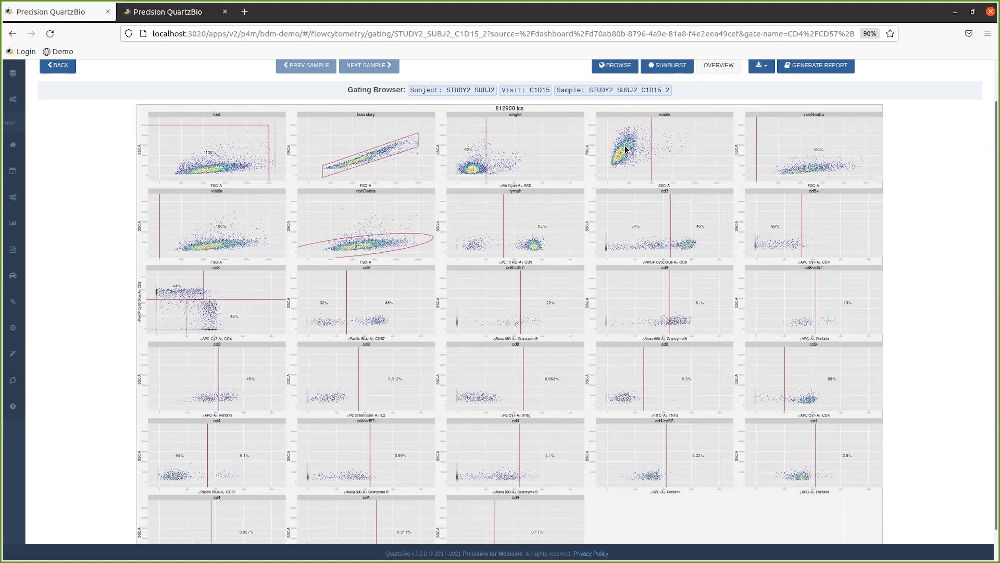
Our client’s translational research team needed to be able to monitor clinical outcomes with respect to patients and biomarker-defined subgroups. Within weeks, QuartzBio deployed virtual Sample Inventory Management and Biomarker Data Management solutions for the clinical trial in progress, enabling the team to:
- Gain centralized visibility into status of samples as they move from sites to central and specialty labs
- Reveal insights on actual samples collected versus expected samples collected
- Generate hypotheses based on biomarker assay results linked to patient clinical data and dosage information
- Eliminate time-consuming and manual data cleaning and cross-referencing processes

